Rank the following acids from lowest pka to highest pka. – Rank the following acids from lowest pKa to highest pKa: Rank acids based on their pKa values to determine their relative strengths. This comprehensive guide delves into the concept of pKa, exploring its significance and the factors that influence it, providing a step-by-step approach to ranking acids accurately.
pKa, a crucial parameter in acid-base chemistry, measures the acidity of an acid, quantifying its ability to donate protons. Understanding pKa is essential for comprehending acid-base reactions and their applications in various fields.
1. Understanding pKa
pKa adalah ukuran kekuatan asam yang menyatakan konsentrasi ion hidrogen (H+) dalam larutan. Semakin rendah nilai pKa, semakin kuat asamnya.
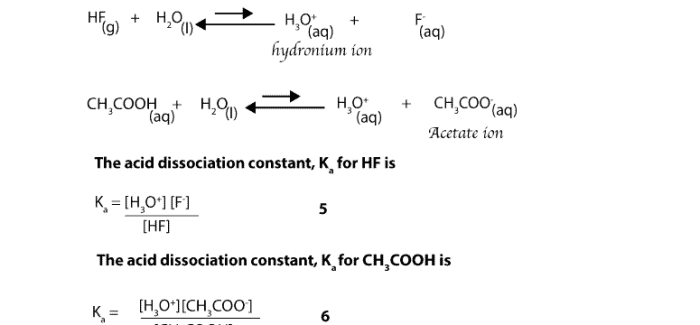
pKa didefinisikan sebagai logaritma negatif dari konstanta disosiasi asam (Ka):
pKa =
log(Ka)
2. Factors Influencing pKa
Faktor-faktor yang mempengaruhi pKa meliputi:
- Elektronegativitas:Semakin tinggi elektronegativitas atom pusat, semakin kuat asamnya (pKa lebih rendah).
- Resonansi:Resonansi menstabilkan ion konjugat basa, sehingga menurunkan pKa (asam lebih lemah).
- Efek Induktif:Gugus penarik elektron (seperti -NO2) meningkatkan pKa (asam lebih lemah), sedangkan gugus pendorong elektron (seperti -OCH3) menurunkan pKa (asam lebih kuat).
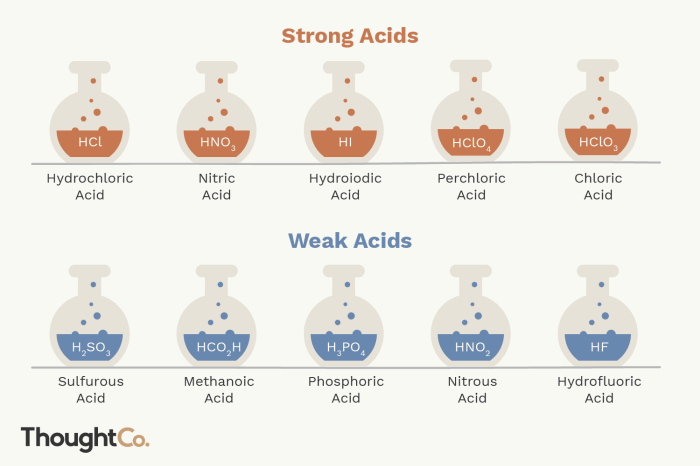
3. Ranking Acids by pKa: Rank The Following Acids From Lowest Pka To Highest Pka.

Untuk mengurutkan asam dari pKa terendah (terkuat) ke tertinggi ( terlemah), ikuti langkah-langkah berikut:
- Tentukan pKa masing-masing asam.
- Bandingkan nilai pKa: asam dengan pKa terendah adalah asam terkuat.
- Urutkan asam dalam urutan menaik pKa, dari asam terkuat ke terlemah.
Q&A
What is the relationship between pKa and acid strength?
Acids with lower pKa values are stronger acids, as they dissociate more readily, releasing more protons into the solution.
How does electronegativity influence pKa?
Electronegative atoms, such as oxygen and fluorine, stabilize the conjugate base of an acid, lowering its pKa and increasing its acidity.
Can resonance affect pKa?
Resonance can lower the pKa of an acid by delocalizing the negative charge of the conjugate base, making it more stable.
